Tepezza, a prescription medication approved by the U.S. Food and Drug Administration (FDA) for the treatment of thyroid eye disease (TED), has gained recognition for its efficacy in managing symptoms associated with this debilitating condition. However, recent studies have shed light on uncharted risks that may impact both wellness and hearing.
Drugwatch reports that, according to a study with 26 people who got at least four Tepezza injections, roughly 23% of the subjects reported hearing loss. The authors of the research highlighted that the hearing loss was either new or worsening after using the medicine.
In this article, we will discuss the potential dangers posed by Tepezza, exploring its unforeseen effects and the concerns they raise.
The Promise and Peril of Tepezza
Tepezza, also known as teprotumumab-trbw, was hailed as a breakthrough treatment for TED. It acts at the source, blocking autoantibodies responsible for triggering the disease’s progression. The drug effectively reduces inflammation, prevents tissue expansion, and inhibits muscle and fat tissue remodeling. Despite its initial success, the emergence of unanticipated risks calls for further investigation.
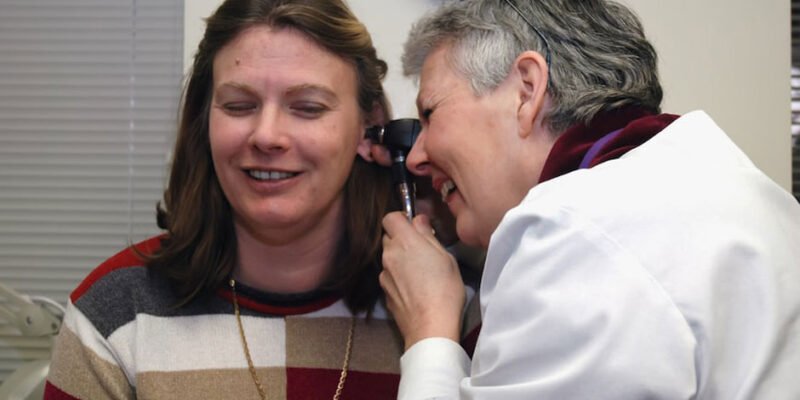
Hearing Loss and Tinnitus
Intriguingly, studies have revealed a potential link between Tepezza and hearing-related problems. While early trials did not report such issues, subsequent research uncovered a higher incidence of hearing loss, tinnitus, and other auditory conditions among patients who received the medication. These unexpected side effects have sparked concern and raised questions about the drug’s safety profile.
The Endocrine Society Study
A pivotal study conducted by the Endocrine Society in March 2021 uncovered alarming statistics. Among the participants who received Tepezza, a significant number experienced subjective hearing loss, ringing in the ears, a feeling of plugged ears, and heightened perception of their own voice.
Some even developed patulous eustachian tube dysfunction, which distorts vocal sounds. These findings underscore the need for comprehensive assessment and further research.
Impact on Quality of Life
Thyroid eye disease is already associated with a range of distressing symptoms, including pain, double vision, and watery eyes. The addition of hearing-related problems compounds the challenges faced by individuals with TED, significantly affecting their quality of life. Increased sensitivity to sound, muffled hearing, and the sensation of plugged ears further contribute to the burdensome nature of the disease.
The cumulative impact of these hearing-related issues on the quality of life of individuals with TED cannot be overstated. The combination of already challenging TED symptoms with these additional complications places a substantial burden on affected individuals, highlighting the need for a thorough evaluation, early intervention, and appropriate compensation for the harm caused by Tepezza.
Pursuing Compensation and Justice
For those who have experienced hearing-related issues after taking Tepezza, seeking legal recourse through a Tepezza Lawsuit may be an option. The concept of failure to warn, a type of product liability claim, comes into play when a manufacturer fails to adequately disclose potential risks associated with their product.
According to a recent update by AboutLawsuits, the lawsuits were recently merged into a multidistrict litigation (MDL) before Judge Thomas Durkin of the Northern District of Illinois. Judge Durkin is anticipated to adopt a first case management plan, allowing the parties to continue forward with discovery into the relationship between Tepezza and hearing loss.
According to TruLaw, engaging with an experienced defective drug lawyer can help affected individuals navigate the legal process and pursue compensation for their injuries and losses. While the compensation amount varies based on the specifics of each case, it may cover various aspects such as medical expenses, lost wages, pain and suffering, and other related damages.
The Importance of Informed Decision-Making
While the risks associated with Tepezza may be concerning, it is essential to weigh them against the potential benefits the drug offers in managing thyroid eye disease. Open communication between patients and healthcare providers becomes crucial for making informed treatment decisions.
As ongoing research expands our understanding of Tepezza’s risks, maintaining a vigilant approach to monitoring and addressing potential side effects becomes paramount.
Final Word
The recent studies highlighting the potential risks of Tepezza to hearing wellness have raised significant concerns. The unexpected occurrence of hearing loss and other auditory conditions among patients who received the medication warrants further investigation into its safety profile.
These unforeseen side effects pose additional challenges to individuals already coping with the symptoms of thyroid eye disease, impacting their quality of life. Seeking legal recourse through a Tepezza Lawsuit may be an option for those affected.
Moving forward, it is crucial for healthcare providers and patients to engage in open communication and make informed decisions regarding the use of Tepezza, considering both its potential benefits and risks.












Comments